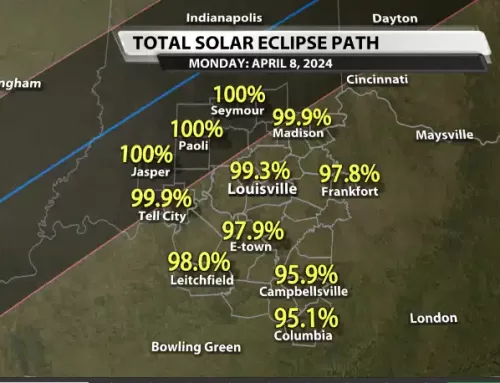
By Matthew Keck–
Thomas E. Dunbar made a $1 million donation to the University of Louisville James Graham Brown Cancer Center on Oct. 15. This donation will go towards the creation of a specialized center to provide chimeric antigen receptor positive T-cell (CAR T-cell) therapies for patients.
“The latest and best treatments for cancer don’t involve chemotherapy and radiation therapy,” said U of L department of surgery chair Dr. Kelly McMasters. “They involve immunotherapy; using the bodies own immune system to attack the cancer.”
T-cells are part of the immune system and help protect the body from infection and may help fight against cancer, according to the National Cancer Institute. CAR T-cells are those that are isolated from a patient’s blood and then genetically modified to destroy cancer cells more effectively.
The modified cells are infused back into the patient so that they can fight the cancer and create a long-term immunity in their system. Not only have CAR T-cells showed dramatic treatment results, but this type of treatment also leads to fewer toxic side effects than chemotherapy.
How CAR T-cells work, according to the U of L James Graham Brown Institute:
- A non-infectious virus is used to insert genes into the T-cells that express a receptor specific to proteins, or antigens, present on the cells of the cancer to be treated.
- The armed, loaded T-cell is drawn into close proximity to the cancer cell, and the new cell sends a signal for the T-cell to kill the cancer cell.
CAR T-cell therapy is FDA approved and treats patients with B-cell acute lymphoblastic leukemia, which is more common among children, but also adults with non-Hodgkin’s lymphoma. They will be testing this type of treatment for other cancers through clinical trials. Until now, this kind of treatment was only available in larger coastal cities.
With formation of the CAR T-cell program there will be laboratories that manufacture the treatment, along with administering clinical-trial therapies to adult and pediatric patients. This program will conduct trials and research on the effects that CAR T-cell therapy has on other types of cancer in Louisville.
Facilities for this program are known as Good Manufacturing Practice (GMP) laboratories, and they require specialized documentation and equipment to protect the individuals working there. These rooms require a specialized clean and sterile environment for manipulating patient’s immune cells.
The Dunbar CAR T-Cell program will have two GMP labs, one for pediatric therapies and the other for adult. The pediatric lab will be named after his late son Evan Dunbar, and the adult lab will have the title Altobellis, after his wife Dr. Stephanie Altobellis. Each of these labs intends to extend their reach not only to Louisville, but across the state of Kentucky and the Midwest.
Dunbar lost his son Evan to neuroblastoma in 2001 at the age of 6 and his father, Wally Dunbar, to melanoma in 2009. His wife, Dr. Altobellis, helped identify his own cancer this year. “My frustration comes from, why didn’t I do more, sooner,” said Dunbar.
The goal of this program is to improve treatment for cancer patients. They plan is to have the facilities fully functional and taking patients by Sept. 30, 2020.